Designing an Open-Source Flow Battery Kit
Flow Battery Research Collective
Flow Battery Research Collective
Utrecht University/FAIR Battery Project
Utrecht University/FAIR Battery Project
April 9, 2024
Flow Battery Research Collective: Who are we?
Researchers who want to help develop and democratize technologies for affordable, sustainable energy storage
What did we do?
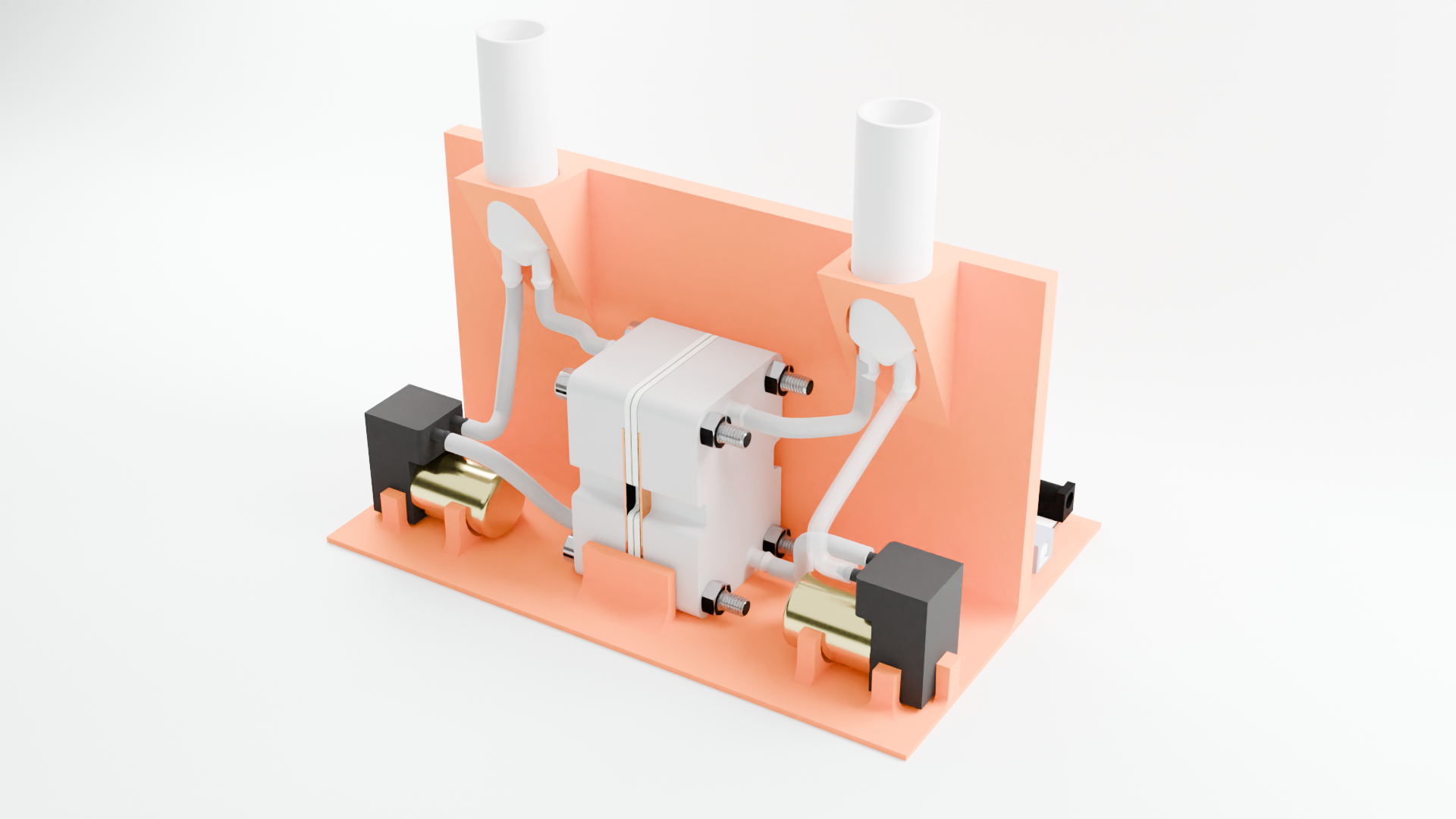
Built an affordable, complete kit which allows small-scale testing, using:
existing open-source tools: FreeCAD software, MYSTAT potentiostat (Irving, Cecil, and Yates 2021)
non-proprietary components: paper separators, graphite foil
Why did we do it?
- Commercial test kits are expensive
- Existing academic designs aren’t yet accessible to non-experts: lacking documentation and everything needed to perform experiment
- We want to see for ourselves if these technologies work
Commercial cells
ElectroCell

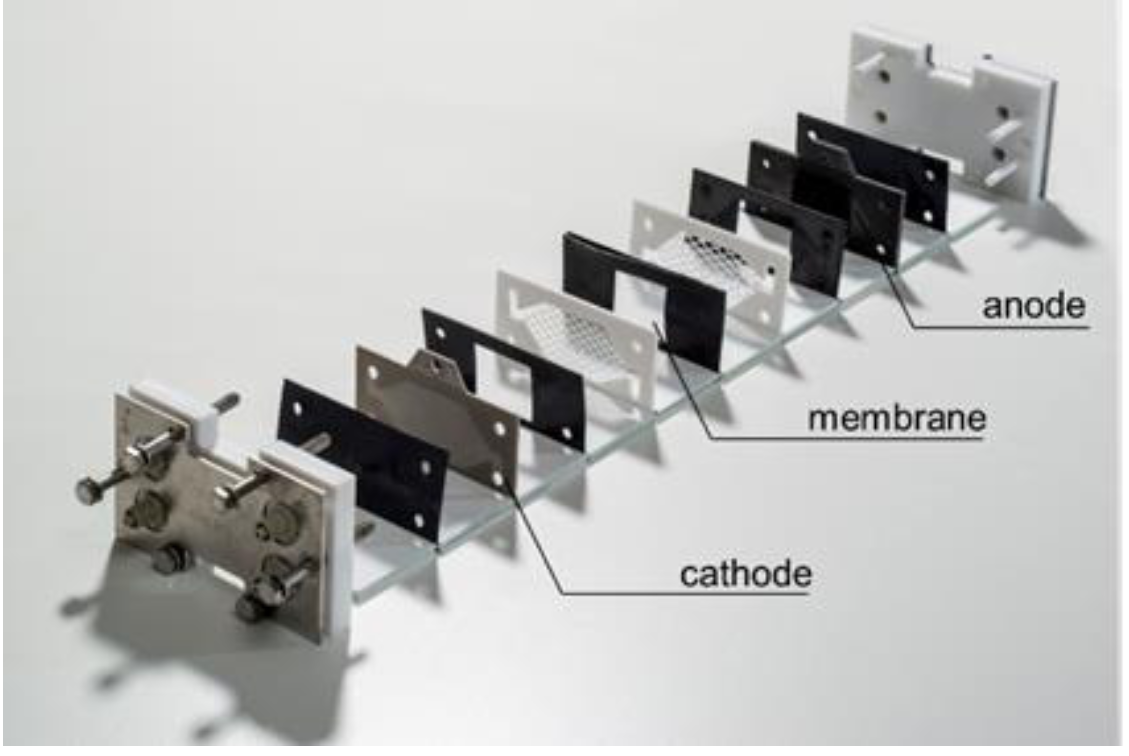
Electrocell (cont.)
✅ Pros:
- Highly configurable
- Simple sealing principle
- Internal manifolding
❌ Cons:
- Machining of metal and specialized graphite material
- Price
C-Tech Innovation

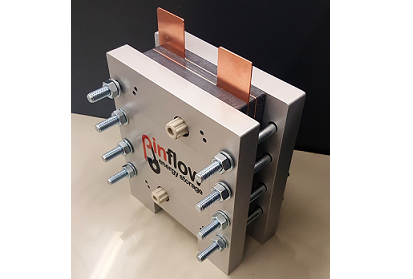
Pinflow Energy Storage > $ 7,000 USD
Redox-Flow.com > € 2,900
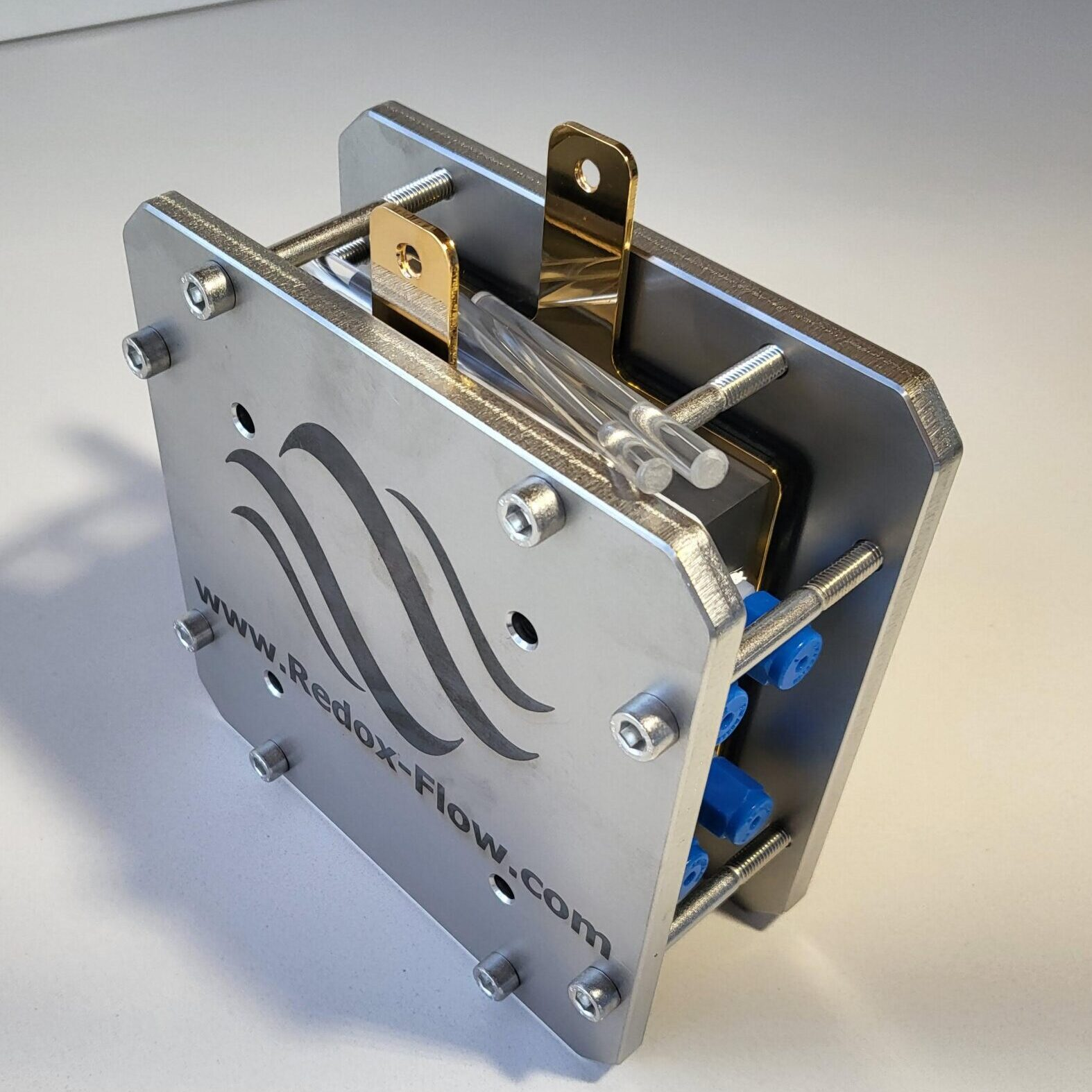
Note the internal manifolding
“Redox-Flow.com: A-Cell, Redox Flow Battery Test Cell” (2024)
MTI Corporation: > $ 9,000 USD


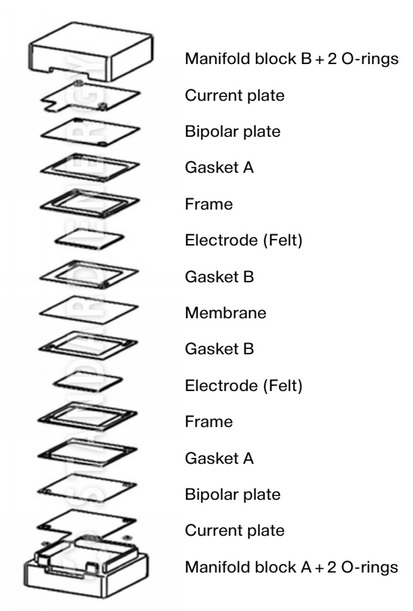
Scribner > $ 5,000 USD

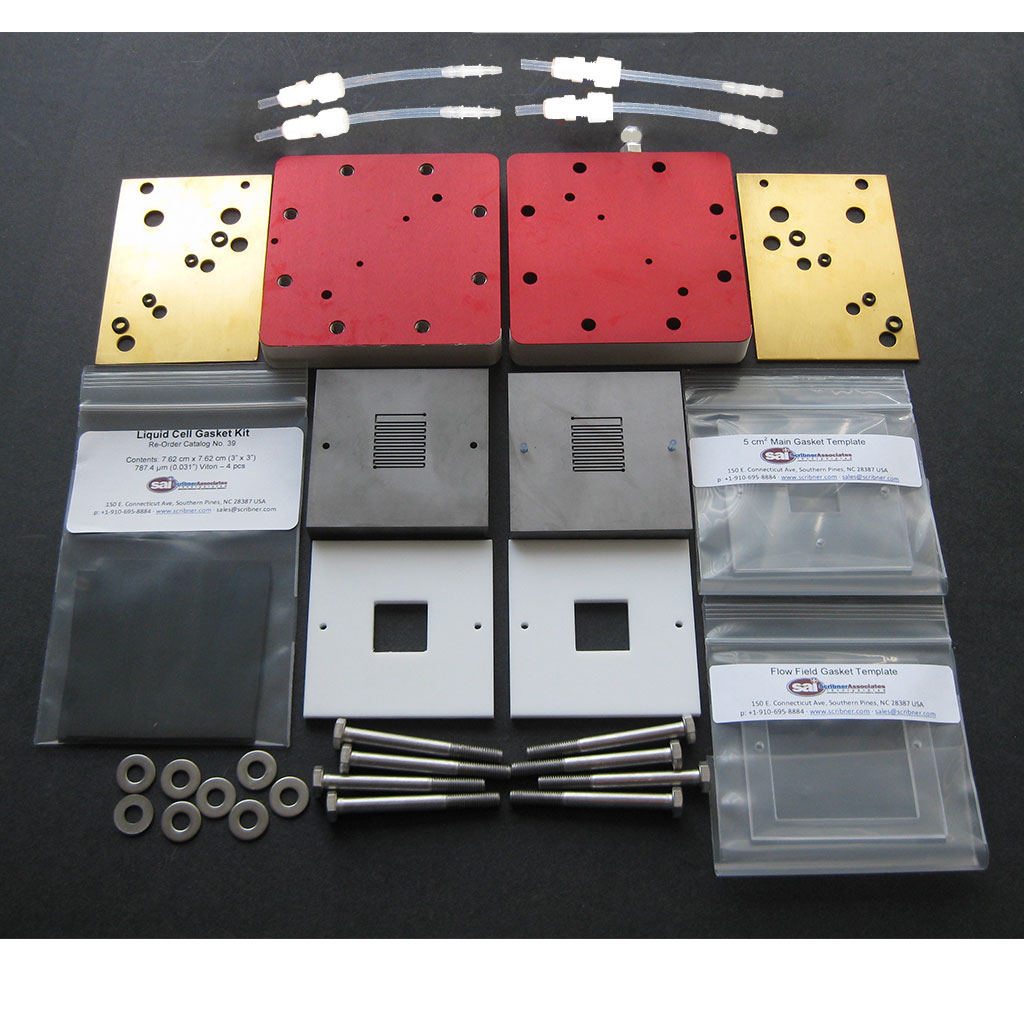
General observations of commercial test cells
Not cheap
Pricing not transparent (two exceptions)
Sturdy: thick metal plates
Requires machining of metal and graphite plates
Electronics/pumps not always included
Academic cells
Companies may buy those commercial cells, academic researchers often make their own.
Here are some of their designs.
QUILL cell
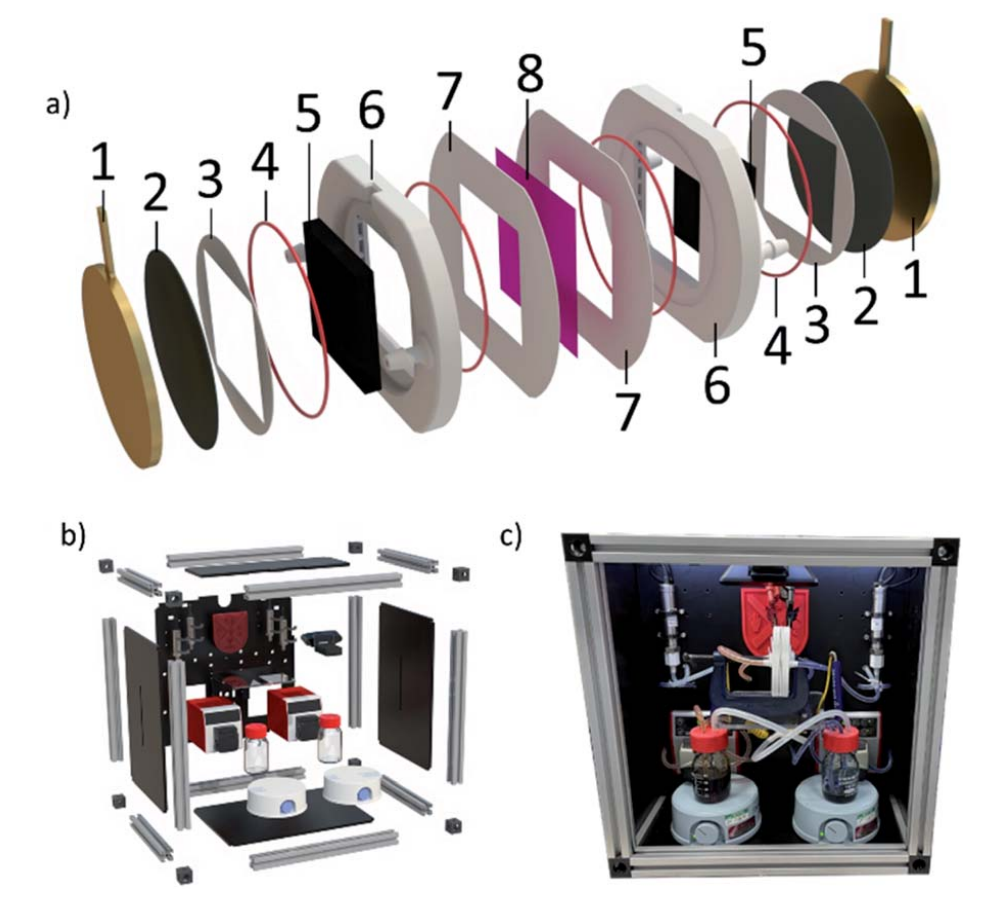
Tested with vanadium chemistry
Good overview of existing cell designs in Supporting Information
QUILL cell (cont.)
✅ Pros:
- Open source!
- 3D printable cell design
- Improved performance vs. commercial C-Tech cell
- Cell fabrication and assembly documentation
- Ergonomic, closes with C-clamp
- Minimal graphite machining needed
- Flow enters electrodes in-plane with membrane/electrode1
❌ Cons:
- Cell design cannot be milled (overhanging geometry)
- Large cell pitch of ~8 mm (distance between current collectors)
- Doesn’t include balance-of-plant
- Couldn’t seal polypropylene cells (only ABS)
MIT Cell (Brushett Group)
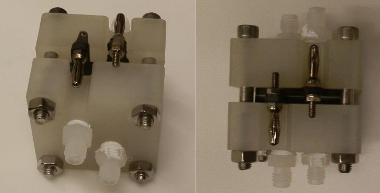
MIT Cell (cont.)
✅ Pros:
- Open-source!
- Well-documented
- Machinable from solid polypropylene
- Capable of small interelectrode distances / cell pitch
❌ Cons:
- Requires milling of specialized graphite plate material
- Flow enters electrode perpendicular to electrode/membrane plane
- Fixed size at ~2 cm2
Southampton Cell
Successful demonstration of 3D-printed cell components, using zinc-cerium chemistry. Similar approach to Electrocell (Arenas, Walsh, and León 2015)
General observations of academic test cells
- Varying degrees of openness/documentation
- Use of additive manufacturing methods common
- Doesn’t include entire, reproducible system
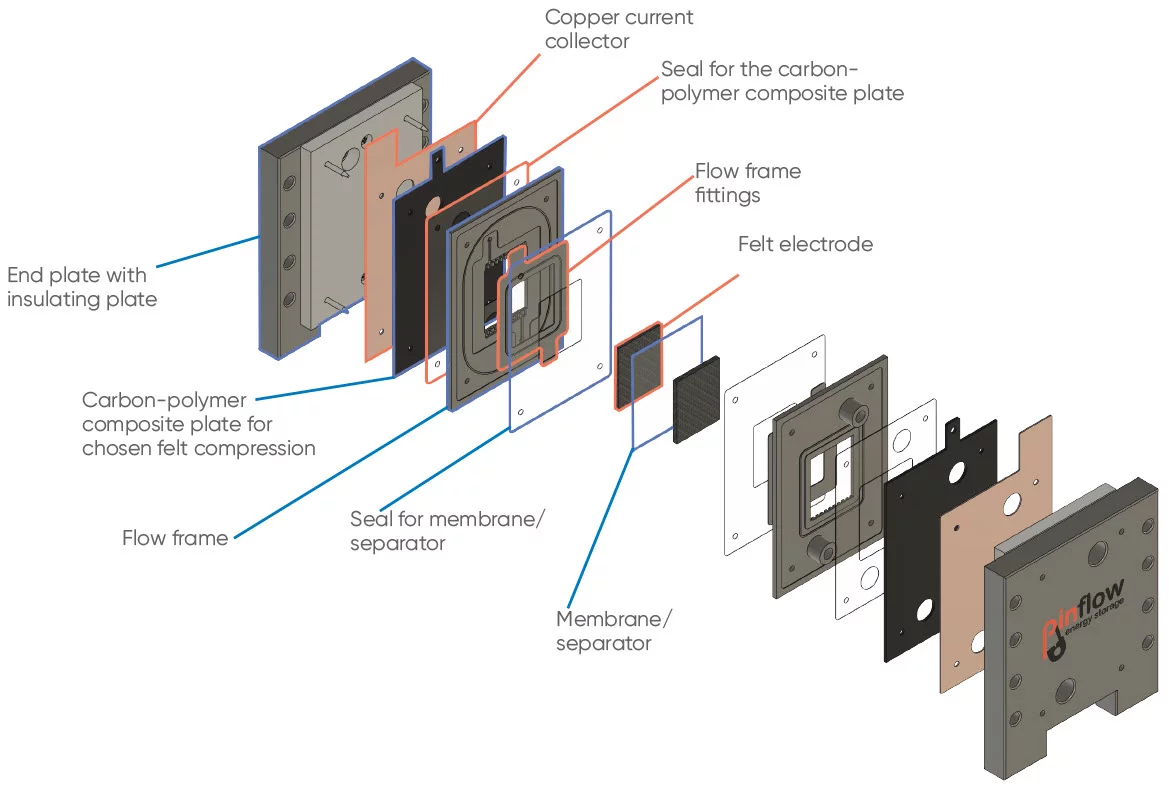
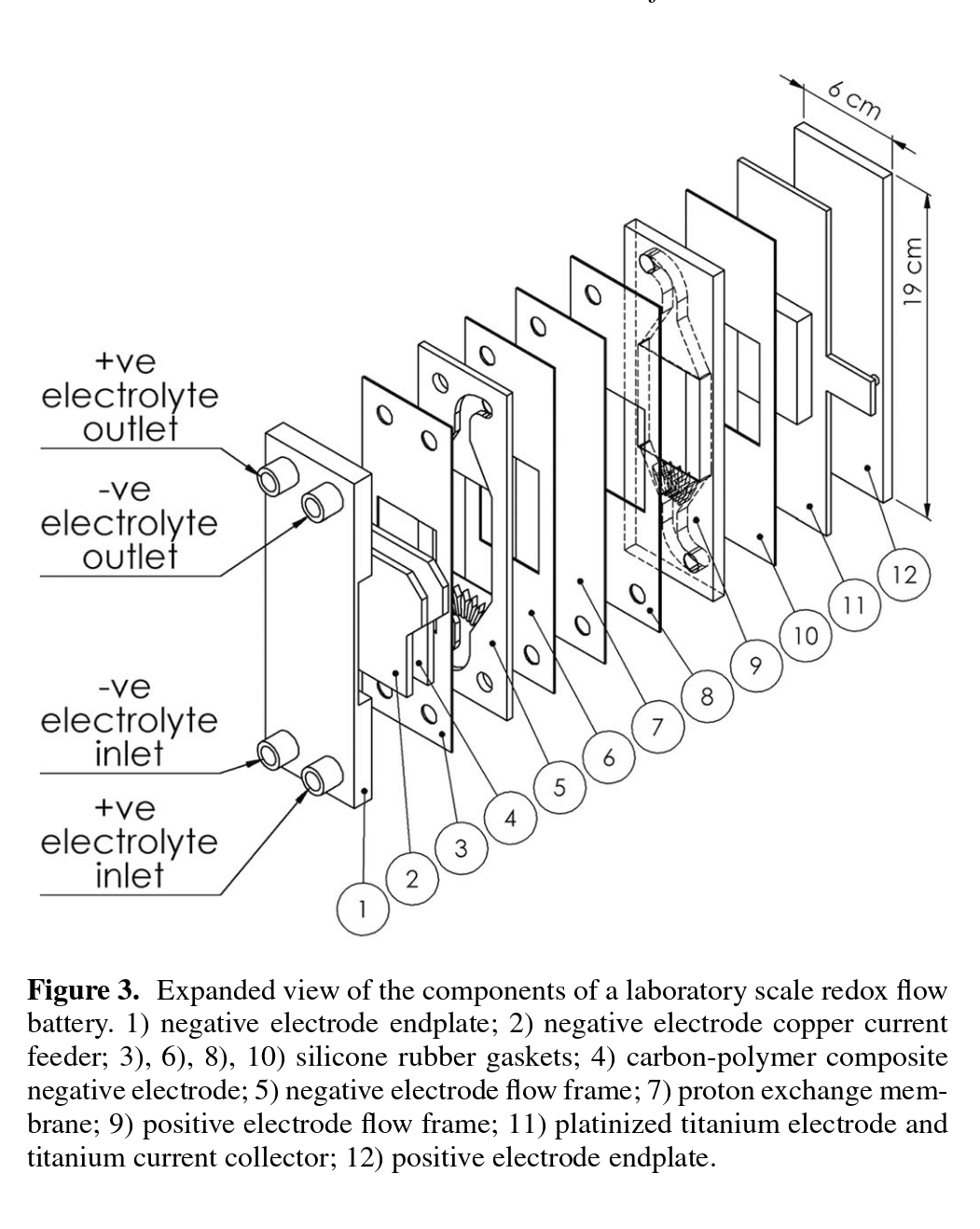
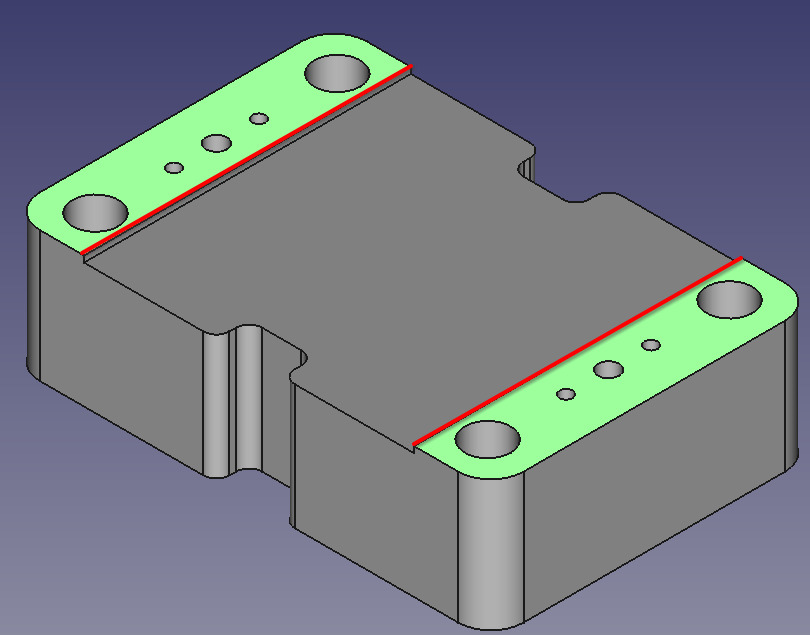
Our design
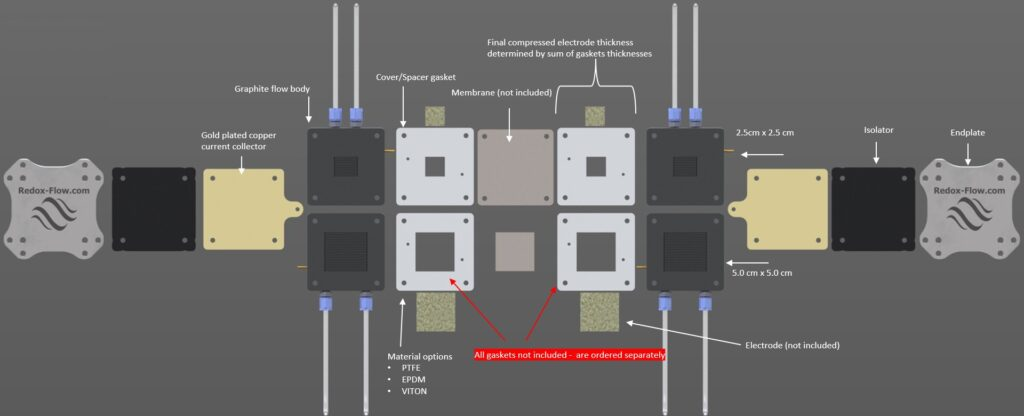
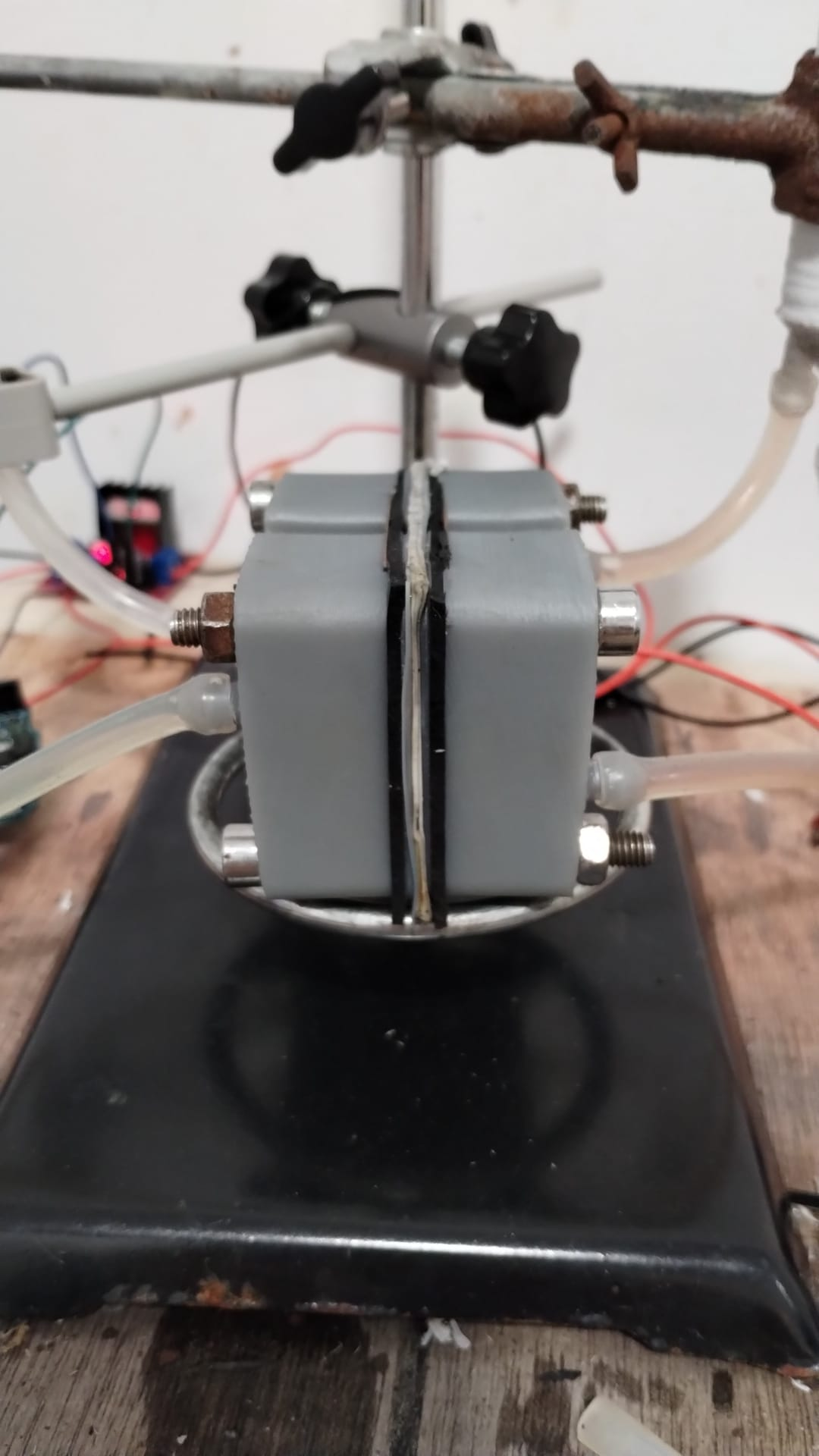
Exploded view of current design
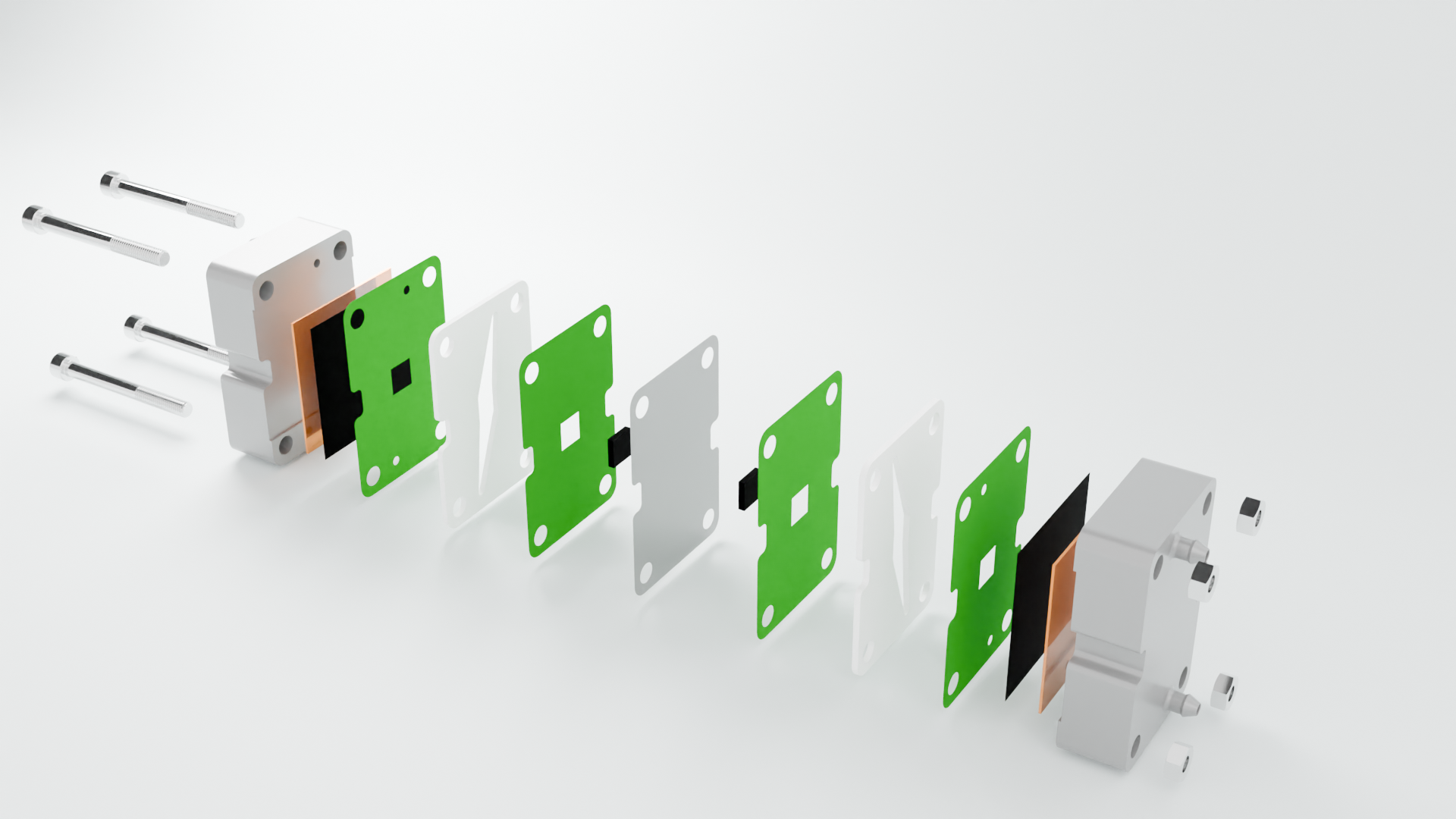
Exploded view of the current cell design
Affordable membrane

2,520 EUR/m2 vs. 6 EUR/m2 (Ion Power GmbH and Amazon UK)
Affordable current collectors

1,477 EUR/m2 vs. 66 EUR/m2 (FuelCellStore and Amazon US)
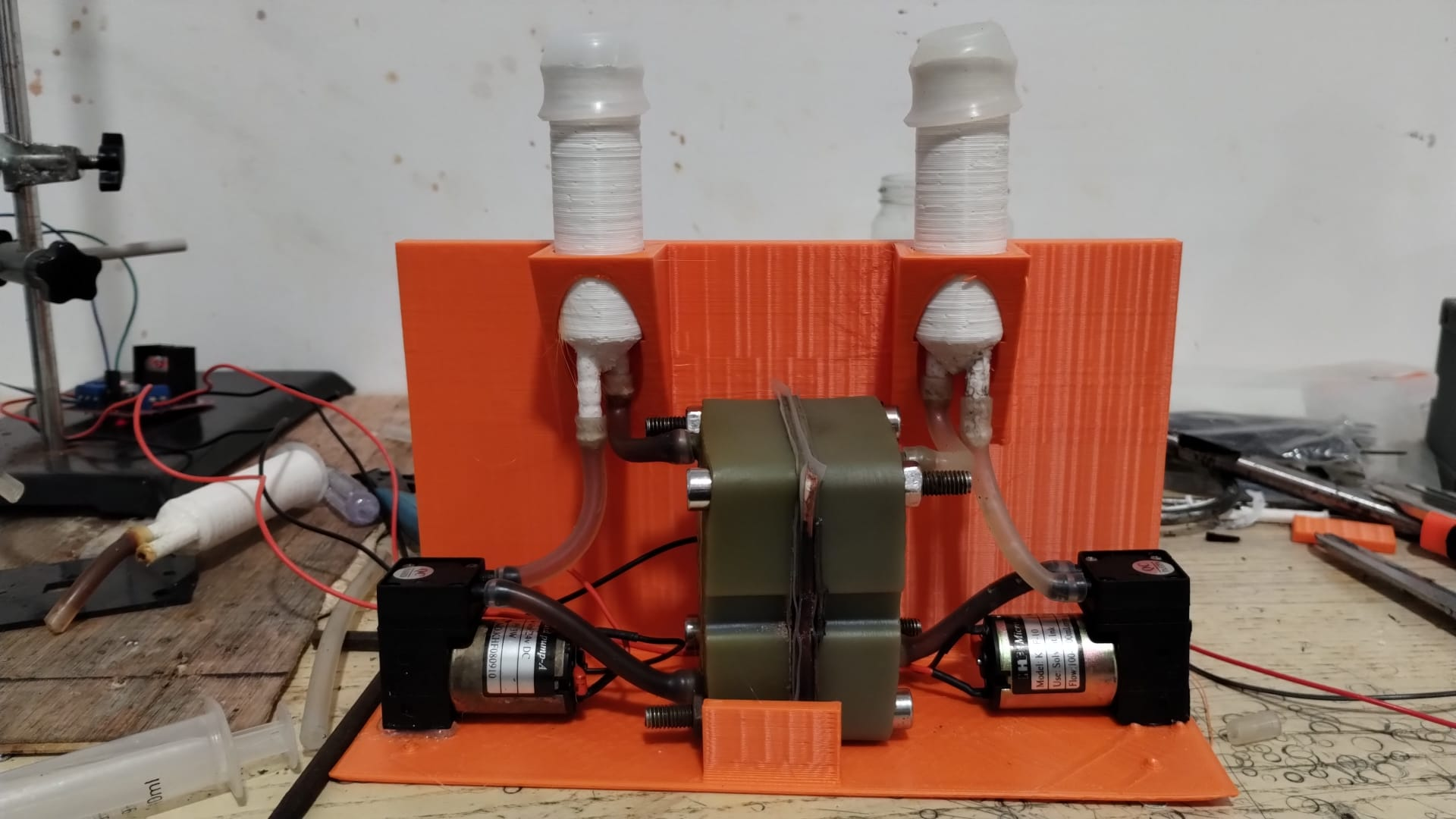
Our design: pros and cons

✅ Pros:
- Off-the-shelf parts, including graphite foil used on top of copper current collector
- 1 cm2 to 10 cm2 area configurable
- Possibility for small interelectrode gap
- Flow enters porous electrode in-plane with membrane
❌ Cons:
- In practice, 3D-printable… but machined cell bodies are best
- Precise fit required between copper current collectors and plastic cell body
- Thick plastic required in absence of metal compression plates
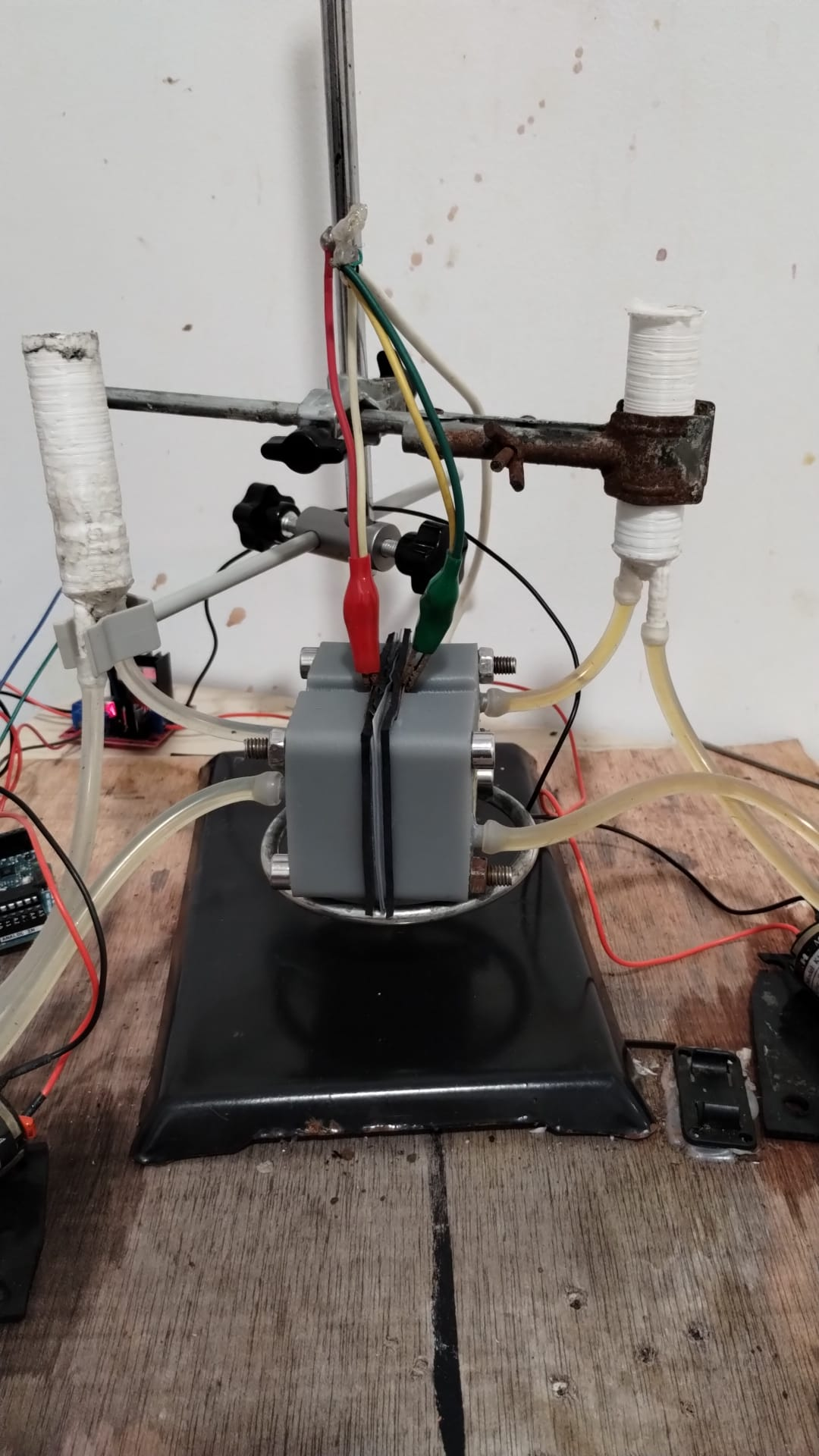
Entire kit, front

Jig holds everything in place
- Affordable diaphragm pumps
- Flexible tubing and barbed fitting connections
- 3D-printable reservoirs with integrated fittings, standpipe, and septum closure
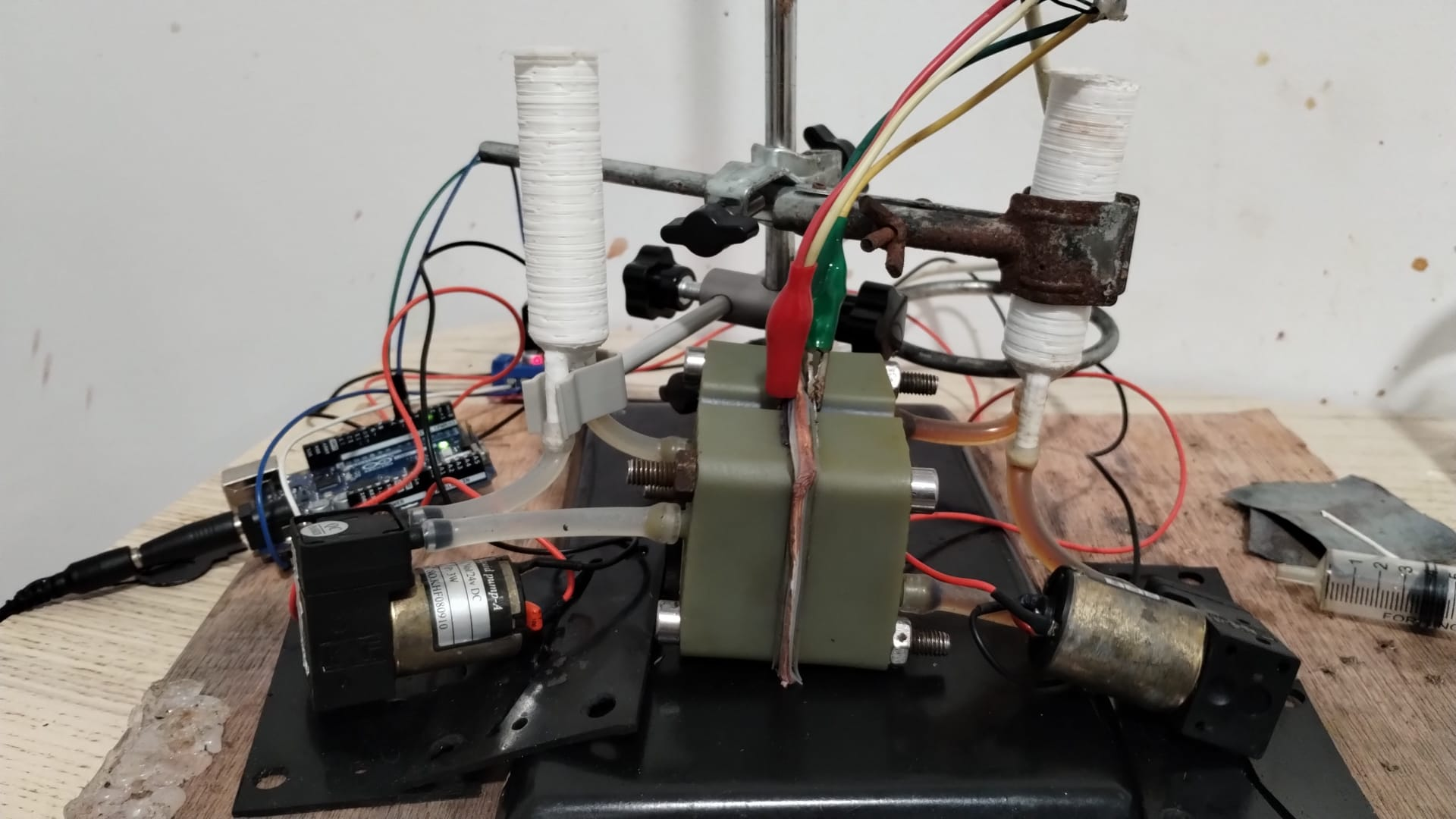
Entire kit, back
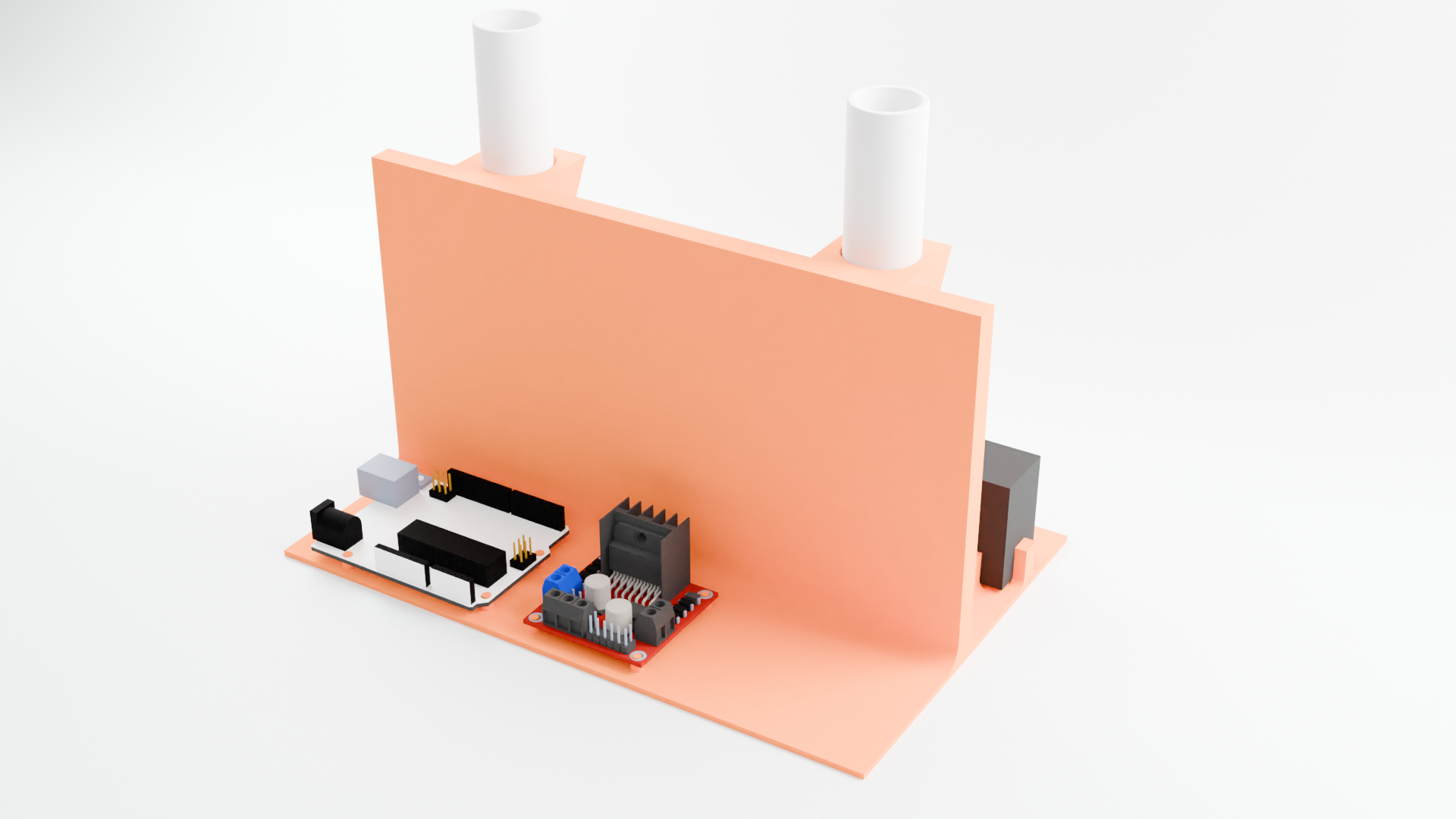
Arduino and motor driver to control pump speeds (potentiostat not pictured)
Prototyping: FDM printing…

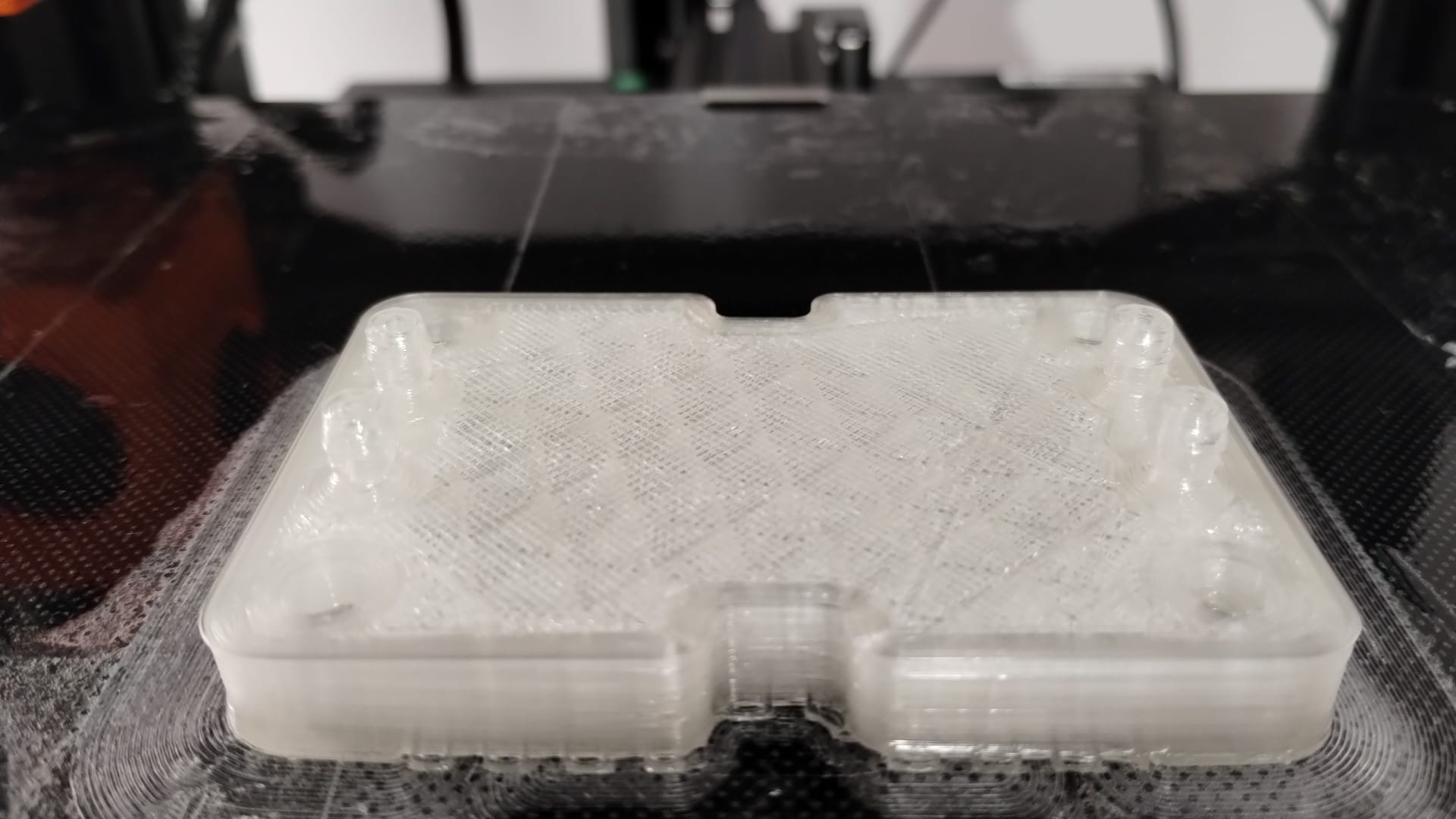
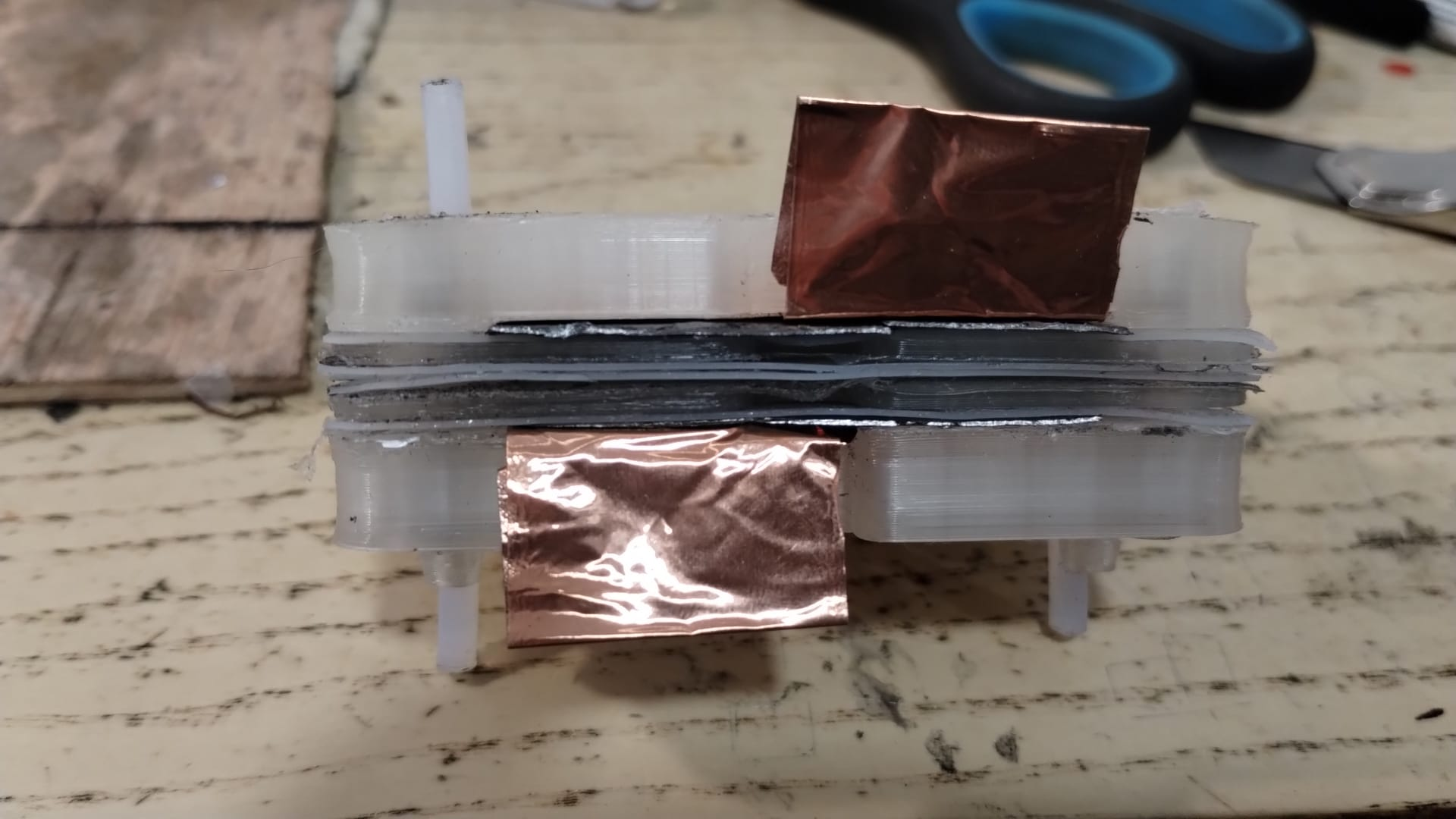

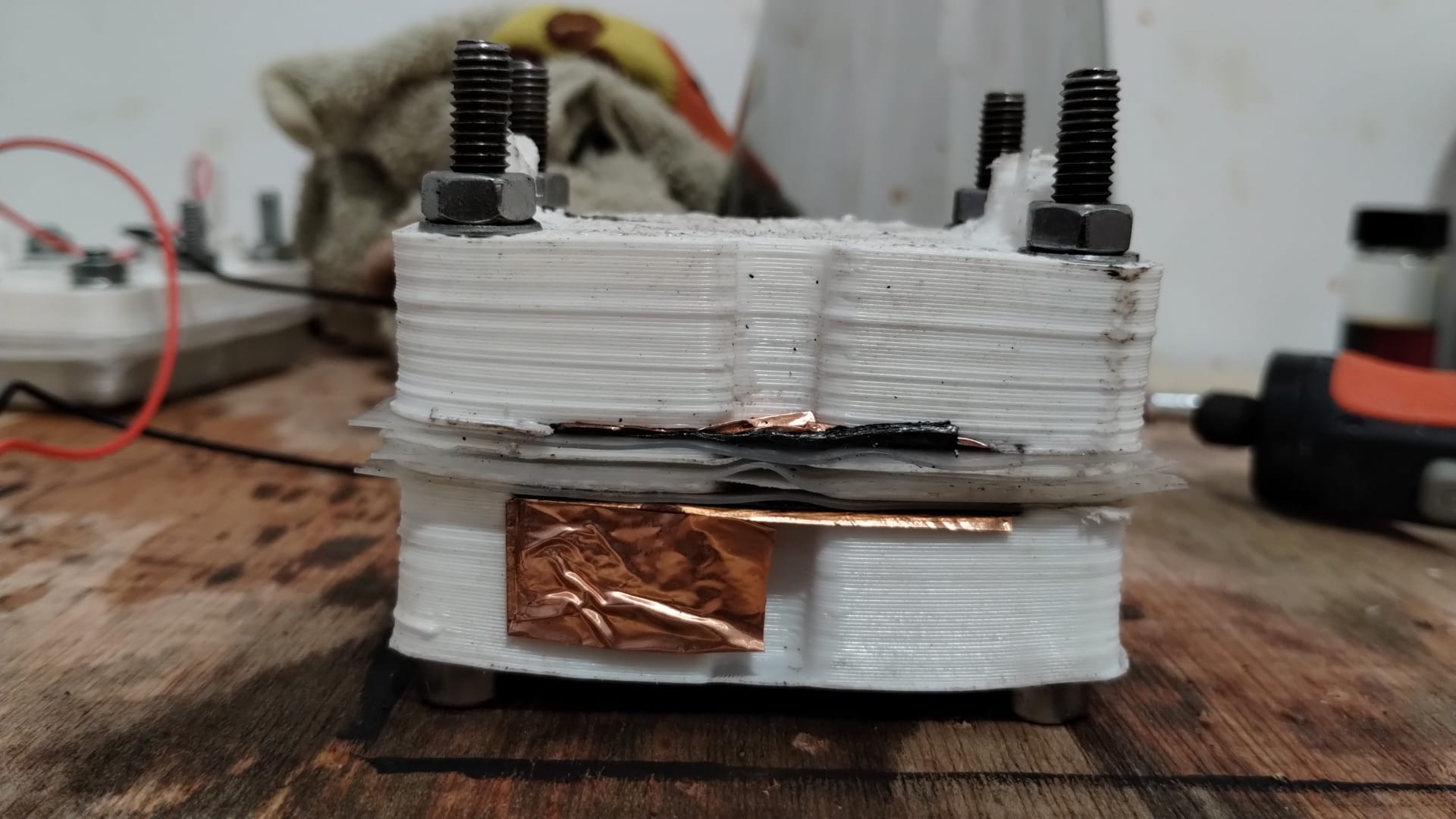

Resin printing


The fancy version you’re getting…

The software
The chemistry
Anolyte: \(\ce{3 Zn^2+ + 6e- -> 3Zn_{(s)} }\)
Catholyte: \(\ce{6I- -> 2I3- + 6e-}\)
Overall: \(\ce{3Zn2+ + 6I- -> 3Zn_{(s)} + 2I3- }, E^\ominus = 1.298 V\)
Parasitic reaction: \(\ce{6I- + O2 + 2 H2O -> 2I3- + 4OH- }\)
Why Zn/I?
Easy to source reagents
Low-cost reagents
Compatible with microporous membranes
Works with readily available paper as membrane
Dendrites are a non-issue with microporous membranes
No detectable hydrogen evolution
Acceptable energy density (>20Wh/L)
No strong acids or bases needed
Low toxicity (but don’t drink it)
Current performance
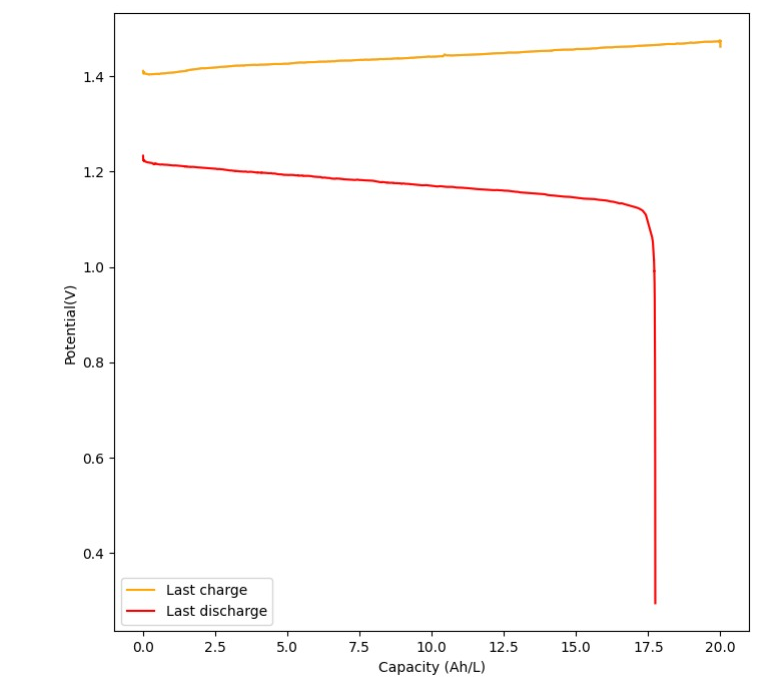
Coloumbic efficiency: 88%
Energy efficiency: 71%
Accessible energy density: 8.9 Wh/L
Avenues to explore
- Hydraulic flow from anode to cathode across microporous membrane
- Durability of separator membrane
- Long term cycling
- Use of other microporous separators (Daramic for example)
- Modifications of microporous separator (PVA for example)
You can help!
- Documentation
- Cell design
- Electrolyte design
- Growing the community
- Scale-up!
Acknowledgements
- Josh “Bolt Breaker” Hauser, Sanli Faez & FAIR Battery team
- Daniel Fernández Pinto
- Conference organizers!



References
Workshop
Can you put together a cell that doesn’t leak?

Flow4UBattery Event, Eindhoven